Your process data is not only a means of demonstrating compliance for regulatory purposes
It can also be used to give insights into your production processes, identifying areas where improvements may be made.
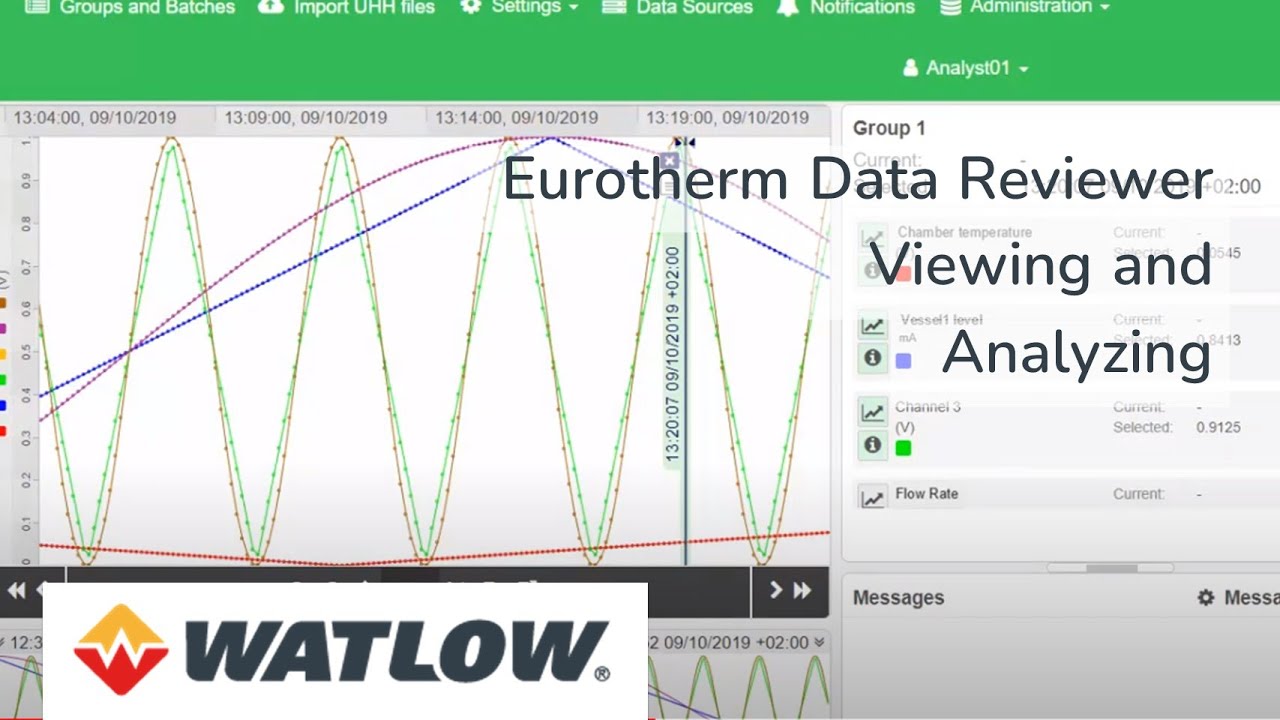
The ability to browse and analyse your process data at any time, without the need for application or database expertise, is essential for agility and continuous improvement.
Eurotherm Data Reviewer empowers you to display, analyse, print and share historical data files, however you want, whenever you want. With the Auditor option, a full audit trail is provided for annotations and excursion information, with user authentication and electronic signatures to facilitate accountability, integrity, and confidentiality of the source data., hoe u maar wilt en wanneer u maar wilt.
With regulations such as FDA 21CFR part 11, ALCOA* and AMS2750, data integrity has never been so important for the pharmaceutical, food and heat treatment industries. Eurotherm Data Reviewer provides support for managing user-defined accounts and passwords. Auditor option provides support for electronic signatures in accordance with FDA 21 CFR Part 11 and Data Integrity ALCOA + concept.
Playlist

1:16
2:38












Reviews (%d)
Er zijn nog geen beoordelingen.